Atomic fluorescence spectroscopy is a technique used in biochemical, medical and chemical applications that require very high sensitivity as well as precision and accuracy. An atomic fluorescence spectrometer is capable of measuring samples containing both hydride-forming elements (such as: Arsenic (As), Cadmium (Cd), Zinc (Zn), Bismuth (Bi), Selenium (Se), Tellurium (Te), Antimony (Sb), Tin (Sn), Germanium (Ge), and Lead (Pb)) as well as Mercury (Hg) at a part per trillion (ppt) level using the unique vapor hybrid generator.
Theory of Operation:
The technique behind atomic fluorescence spectroscopy is similar to atomic absorption spectrometry in that a sample absorbs light at a particular wavelength to promote its electrons from its ground electronic state into an excited state. From this excited electronic state, the electron drops down to a lower electronic state into one of several vibrational levels, emitting a photon in the process, with one of several frequencies. By measuring the intensity of the emitted light at particular frequencies, it is possible to determine the concentration of the element being measured. The technique behind the instrument has a great impact on many applications, especially those concerned with analysis of metals in biological samples, agricultural samples, water, and industrial oils.
Atomic Fluorescence Applications
Theory of Operation:
The technique behind atomic fluorescence spectroscopy is similar to atomic absorption spectrometry in that a sample absorbs light at a particular wavelength to promote its electrons from its ground electronic state into an excited state. From this excited electronic state, the electron drops down to a lower electronic state into one of several vibrational levels, emitting a photon in the process, with one of several frequencies. By measuring the intensity of the emitted light at particular frequencies, it is possible to determine the concentration of the element being measured. The technique behind the instrument has a great impact on many applications, especially those concerned with analysis of metals in biological samples, agricultural samples, water, and industrial oils.
 |
| Block Diagram of an Atomic Fluorescent Spectrophotometer |
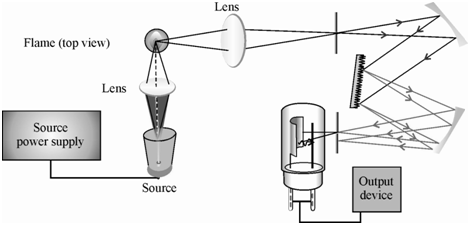 |
| Schematic of an Atomic Fluorescent Spectrophotometer |
Atomic Fluorescence Applications
- Clinical
- Environmental/Public Health/Disease Control
- Agricultural/Food Safety
- Geological/Metallurgical
- Pharmaceutical
- Petrochemical
No comments:
Post a Comment